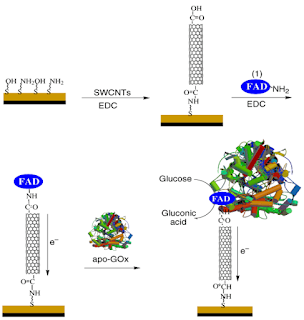
Evaluation of Recent Methods to Improve Recombinant Helicobacter Pylori Protein Yield and Solubility in Escherichia Coli Expression System Successful expression of target genes, often indicated by high yield and solubility, is critical for studies involving recombinant proteins. Yet the most common bacterial expression system utilizing Escherichia coli as host cells is usually reported to produce low amounts of soluble target proteins. In this study, two Helicobacter pylori (Hp) genes, Hp lipase and Hp peptide deformylase (Hp-PDF), whose encoded proteins are crucial for bacterial growth and colonization, thus could be used to screen potential anti-Hp drugs, were designed to be expressed in such system. Genetic engineering, experimental biology, and computational biology methods were employed to enhance recombinant protein production. The result showed that Hp-lipase expression was most improved through construct design that used two restriction enzymes, NdeI and XhoI, in...